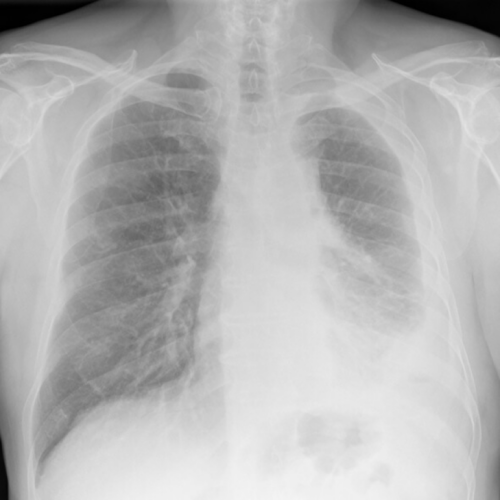
While immunotherapy drugs have shown promise for some patients diagnosed with mesothelioma, not all will respond to the treatments. Unfortunately, biomarkers that can predict drug effectiveness for mesothelioma do not exist. Researchers overseas may help in determining the effectiveness of via electronic technology.
In the Netherlands, scientists are making claims that they have developed a novel breath test for those suffering from the disease as an indicator on how they respond to immunotherapy.
Breath tests may determine responses to immunotherapy
What they refer to as “eNose” can reveal notable differences between patients who have immune checkpoint inhibitors that would better respond to the treatment versus those who don’t have them and could potentially suffer serious side effects.
Their findings followed a study that involved 31 pleural mesothelioma patients who received the combination of Opdivo (nivolumab} and Yervoy (ipilimumab) after the eNose test. The drug combination received approved by the FDA in October of 2020 for “first-line treatment.” It represented a significant milestone for US citizens and their fight against mesothelioma as the first new regimen receiving approval in 16 years.
The European Commission – composed of 27 member states, along with Iceland and Norway – also approved the treatment.
Patients now have options that show more effectiveness than chemotherapy. Initial findings see an overall median survival of 18.1 months for those receiving drug treatments, as opposed to 14.1 months for chemo. Still, side effects remain an issue, particularly those who do not respond to the treatment.
The search for a cure remains a worldwide goal for mesothelioma victims and their family members around the globe. Innovative methods to both test and treat continue towards that path to progress.